
The Greenhouse Effect
Recent news on energy
I'm going to explain the effect known (somewhat erroneously) as the 'greenhouse effect', and what causes it.
As you probably know, sunlight contains a lot of ultraviolet light. When this hits the earth's surface, a fair amount of it turns into infrared.
These infrared rays travel upwards, through the atmosphere.
Now - what happens when an infrared ray hits a gas molecule ?
It depends on what it is. Nitrogen and oxygen are not affected, but water vapour, the third most abundant gas in air, is different.
Look at this graph ... it shows what happens when infrared rays pass through water vapour. The horizantal axis is 'infrared frequency' measured in wavelengths per centimetre. The vertical axis is 'transmittance' and shows how much radiation is transmitted, from 0 to 100%. A dip on the graph means that infrared energy has been absorbed.
The main absorptions are indicated by crosses. You can see that water vapour is quite a good absorber.
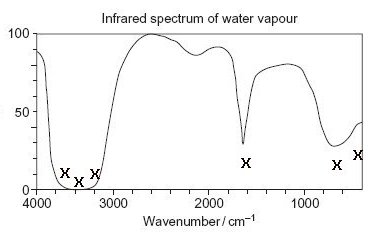
What happens if a molecule absorbs infrared? The answer is - it makes it vibrate. If it's already vibrating, it does it more strongly. The molecule has gained energy.
Now imagine what happens if an air molecule (nitrogen or oxygen) hits that same water molecule.
Some of the extra energy gets transferred to the air molecule. In other words, after the collision, the air molecule moves slightly faster.
It is therefore a little bit warmer.
This is the greenhouse effect.
Forget about greenhouse gases 'trapping' the heat. Gases can't 'trap' anything. That's an explanation designed for kids.
Nearly all of the so-called greenhouse effect is caused by water vapour, because air contains lots of it, especially on damp days; up to about 4%.
Now ... what about carbon dioxide?
The amount of carbon dioxide in the air is much smaller than that of water vapour. It's fifty to a hundred times smaller, depending on the weather.
Here's the graph for carbon dioxide; the same amount of gas as before:
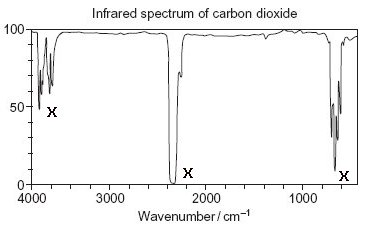
You can see straight away that it's not such a good absorber as water vapour (again, the absorptions are indicated by crosses). More of the infrared rays pass straight through, and the amount of energy absorbed is relatively small.
This leads to the conclusion that carbon dioxide's effect on the air temperature is much smaller than that of water vapour.
It cannot be a significant greenhouse gas.
There are two obvious reasons:
It follows that the effect of carbon dioxide on temperature is slim to zero.
RELATED ARTICLE - DOES CO2 DRIVE CLIMATE?
ND/ habitat21
I am indebted to TG who pointed out that a small temperature rise isn't necessarily trivial. If you are already warm enough, an extra couple of degrees can be very uncomfortable. However, there is strong evidence that the CO2-warming hypothesis is incorrect.
RELATED VIDEO CLIP:
Wasteful 'enviro' schemes: Tim Ball and Ezra Levant; youtube
big turbines
small turbines